TORPEDO Implant System®
The TORPEDO is a spinal implant System who has been designed for:
- Sacroiliac joint dysfunction
- the augmentation, immobilization and stabilization of the sacroiliac joint
- Acute, non-acute, and non-traumatic fractures involving the sacroiliac joint
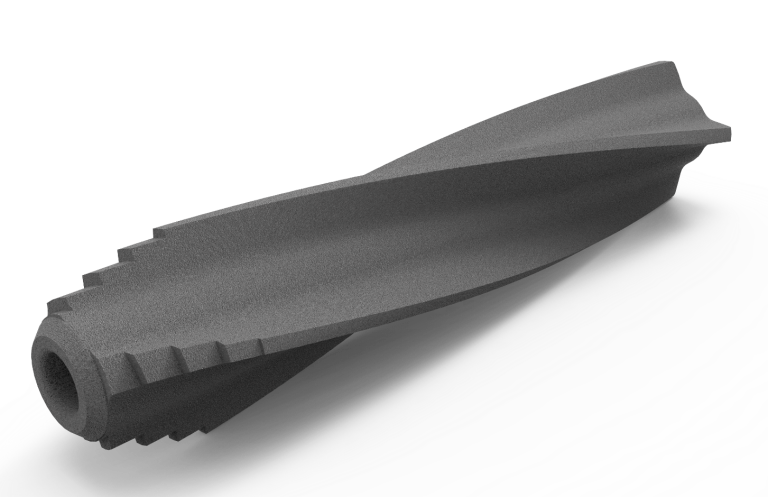
Indications
Sacroiliac joint dysfunction
Sacroiliac joint dysfunction that is a direct result of sacroiliac joint disruption and
degenerative sacroiliitis. This includes conditions whose symptoms began during
pregnancy or in the peripartum period and have persisted postpartum for more than 6
months.
Stabilization of Sacroiliac Joint
TORPEDO is designed for augment immobilization and stabilization of the sacroiliac joint in skeletally mature
patients undergoing sacropelvic fixation as part of a lumbar or thoracolumbar fusion
Fracture involving Sacroiliac Joint
TORPEDO can be indicated for acute, non-acute, and non-traumatic fractures involving the sacroiliac joint.
Female bladder instillation
The UroDapter should be properly installed within the urethral orifice. It is essential to delicately insert the UroDapter into the orifice to guarantee an effective seal, allowing fluid to flow directly into the bladder. By utilizing the UroDapter, there is no requirement for potentially uncomfortable deep insertion methods such as catheter usage. This device enables the direct administration of drugs and other liquids into the bladder, surpassing the need for oral drug administration.
Male bladder instillation
The UroDapter needs to be installed in the urethral orifice of the penis. The UroDapter needs to be put into the so that the fluid find the way straight to the bladder. By using the UroDapter, potentially painful deep insertion using a catheter is not needed. Direct treatment of drugs and other liquid into the bladder can be better applied. It can replace the treatment of drugs orally administered.
Reimbrusement
Hospitals and Private Practices can avail themselves of reimbursement codes specifically designed for bladder instillations involving catheters, which can also be applied to the UroDapter device.
These codes include the:
- CTP-Code: 51700
- HCPCS-Code: A4353
Interested in a free sample?
Contact us!
To request a complimentary sample, kindly complete the form provided. We are pleased to offer this opportunity to experience our product firsthand. Your details will enable us to process your request promptly and ensure accurate delivery. Thank you for considering our offer, and we look forward to providing you with a sample that showcases our expertise and quality.
We need your consent to load the translations
We use a third-party service to translate the website content that may collect data about your activity. Please review the details in the privacy policy and accept the service to view the translations.
